Takeaway
- Majority of patients with non-valvular atrial fibrillation (Afib) starting therapy with non-vitamin K antagonist oral anticoagulants (NOACs: apixaban, dabigatran, and rivaroxaban) were prescribed appropriate daily dose based on the approved European Union (EU) drug label in the UK.
- However, underdosing was twice as frequent among patients starting treatment on apixaban than those on dabigatran or rivaroxaban.
- This is the first and largest assessment for appropriateness of the initial prescribed daily dose of NOACs to patients with non-valvular Afib in the UK.
- Findings warrant research into the patient characteristics that may influence inappropriate underdosing of NOACs.
- Population-based study included 30,467 patients (age, ≥18 years) with non-valvular Afib with a first prescription for NOAC from the UK primary care (January 2011-December 2016).
- Outcome: percentage of patients prescribed a NOAC dose according to the European Union (EU) labels (appropriately dosed), and not according to the EU labels (inappropriately dosed).
- Funding: BAG.
- 50.1% of patients started on rivaroxaban, 35.6% on apixaban and 14.4% on dabigatran.
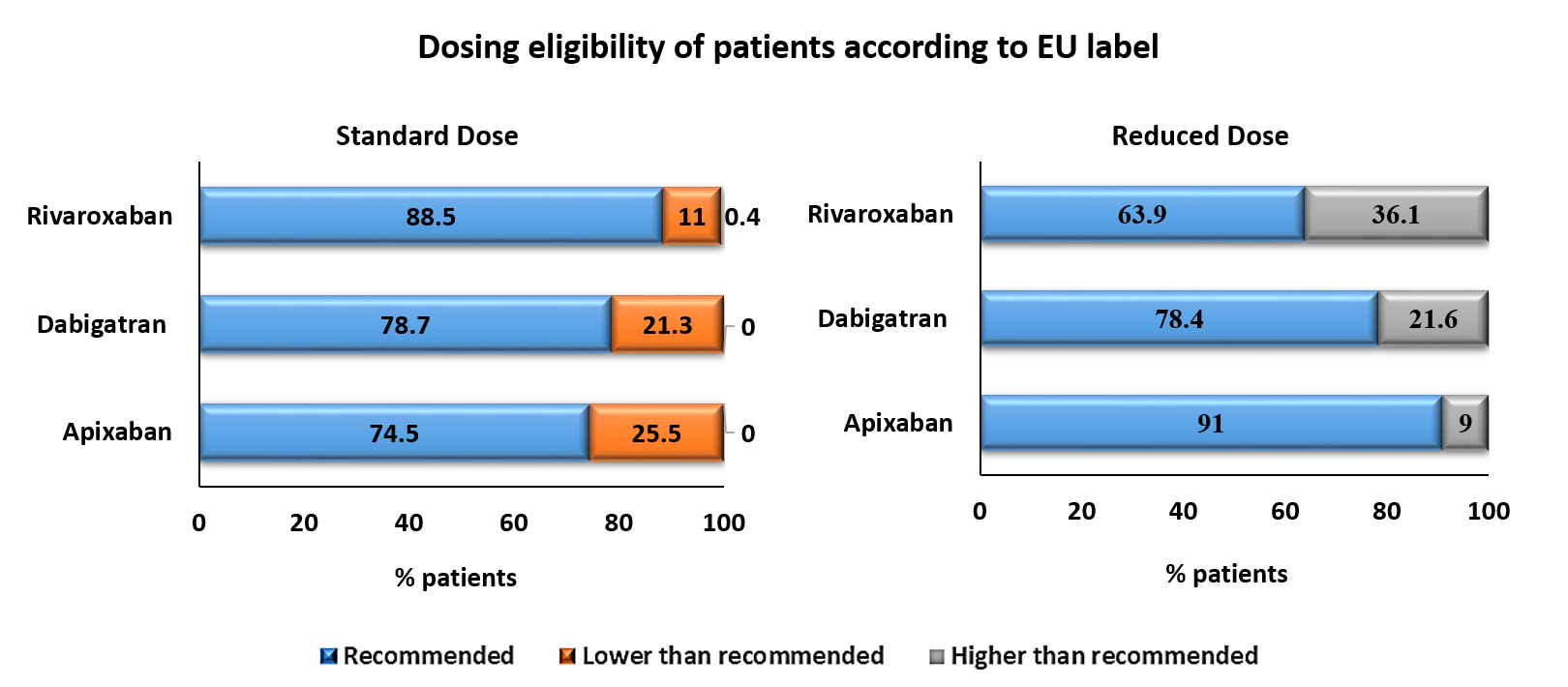
- According to the EU labels, percentage of patients prescribed daily dose of first apixaban, dabigatran and rivaroxaban included:
- recommended dose: 74.9%, 74.4% and 84.2%;
- lower than recommended: 21.6%, 8.7% and 9.1% and
- higher than recommended: 3.5%, 16.9% and 6.6%, respectively.
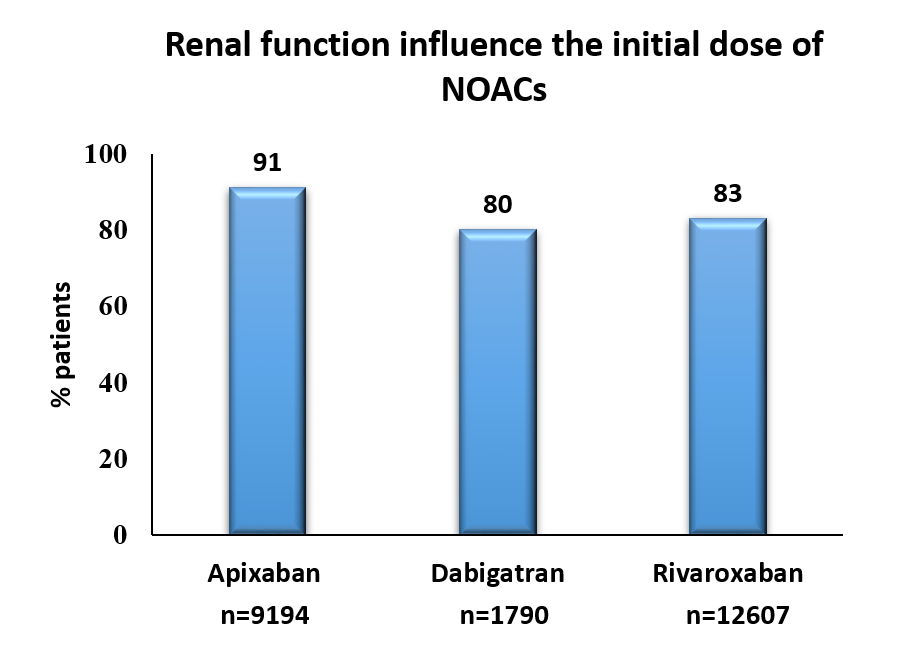
- Patients with severe renal impairment received reduced dose of apixaban (≤5 mg, 91.1%), dabigatran (≤200 mg, 80.0%) and rivaroxaban (15 mg, 83.0%).
Limitations
- Possibility of misclassification for renal function and body weight.
- Potential overdosing may have been miscalculated.
References
References