A British newborn has become the first baby in the world to be enrolled in a cannabis-derived medicine treatment study.
Researchers said it was the first step in trials that could one day lead to a cannabis-derived medicine being used routinely in neonatal care to help babies at risk of seizures and brain injury.
The study will assess whether the medicine is safe and effective in lessening the degree of brain injury for babies with neonatal hypoxic-ischaemic encephalopathy (HIE).
There are currently no approved drugs or medicinal therapies for HIE. Standard care is therapeutic hypothermia, in which the body is cooled to 33.5C.
'I Wanted to Do Everything to Help My Boy'
Oscar Parodi was delivered by emergency caesarean section on 11th March at the Norfolk and Norwich University Hospital (NNUH).
He was unexpectedly born in a poor condition and needed transfer to the Neonatal Intensive Care Unit (NICU) where he was treated with therapeutic hypothermia for 72 hours.
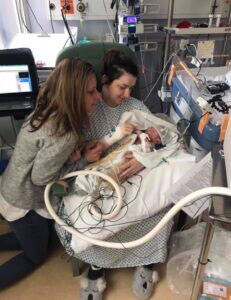
His mother, Chelsea Parodi, of Watton, Norfolk, said: "I was approached after the birth about taking part in this study and I consulted my mum and my brother, who is training to be a paramedic. It was hard but I wanted to do everything I could to help my baby boy."
A small number of other babies in other NICUs in the UK and Europe will join the first phase of the randomised study, which will last a year.
A second baby, born in the same hospital, is also part of the trial.
Babies who take part will continue to receive therapeutic hypothermia. In addition they will receive a single small dose of study medication intravenously, or a placebo, as soon as possible, and within 12 hours of birth.
The therapeutic ingredient of the cannabis-derived medicine occurs naturally in the cannabis plant and is extracted under highly controlled conditions, ensuring the tetrahydrocannabinol (THC) component which causes a 'high' is minimal.
The impact of the trial drug will be monitored by blood samples and measurements of the baby's electrical signals in the brain.
After leaving NICU, the research team will call 30 days, 6 months, and 12 months after discharge from hospital.
'Proud to Have Recruited First Babies'
Prof Paul Clarke, consultant neonatologist at NNUH, said: "There is a lot of excitement on the unit and we are proud to have recruited the very first babies into this study.
"This is the first time a cannabis-derived medicine has been tested intravenously in human babies. It is hoped that it will be good for preventing seizures and protecting the brains of newborn babies with HIE.
“We have always had good support from families wanting to take part in research on our NICU and they often do it from an altruistic point of view to help benefit future babies. One of the attractions of this trial for parents is the closer brain monitoring that babies get as part of the study, because a more advanced brain wave monitor is used for the trial babies. This gives parents more reassurance that any seizures will be picked up."
He added: "As with any study of a new medicine, there may be unexpected side effects and unknown risks.
"With this in mind, the trial has been carefully designed to make it as safe as possible and so we are only giving the babies a minuscule dose at the beginning and we monitor them even more closely than usual."
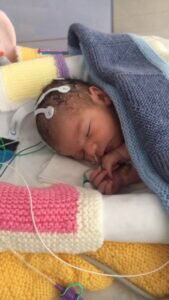
Ms Parodi said: "Oscar was in hospital for 9 days and he was being monitored 24/7. He is doing fantastically well and I am really grateful to Dr Clarke and the team for what they have done for us."
The trial is funded and sponsored by GW Pharmaceuticals, and supported by the National Institute for Health Research.




