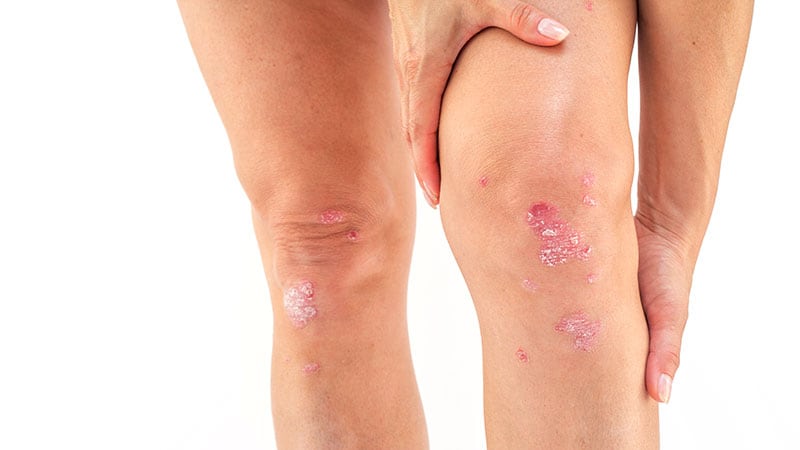
The National Institute for Health and Care Excellence (NICE) has recommended risankizumab (Skyrizi) as a treatment option for moderate-to-severe plaque psoriasis.
Risankizumab is recommended for the treatment of adults with plaque psoriasis if the following conditions are fulfilled:
- Severe disease: Psoriasis Area and Severity Index (PASI) of ≥10 and Dermatology Life Quality Index (DLQI) of >10.
- Inadequate response, intolerance or contraindication to other systemic therapies, including ciclosporin, methotrexate, and phototherapy.
The recommended dose for risankizumab is 150 mg given subcutaneously at weeks 0 and 4 and subsequently every 12 weeks. Therapy should be discontinued if psoriasis has not responded adequately after 16 weeks. An adequate response is defined as a 75 per cent reduction in PASI or a 50 per cent reduction in PASI + 5-point reduction in DLQI.
Evidence has shown risankizumab to be more effective than adalimumab and ustekinumab, and its health benefits may be comparable to ixekizumab and secukinumab. Its cost-effectiveness may be similar to or lower than guselkumab.
The drug is to be used only when provided by the company in agreement with the commercial arrangement. The recommendations should not affect patients whose treatment with the drug was started before the publication of the guidance.