A test that uses earwax to measure levels of cortisol had the potential to "transform diagnostics and care for millions of people with depression", according to results from a small study.
Research results, published in the journal Heliyon, found that earwax samples contained more of the stress hormone than hair samples and could provide a more accurate method to measure chronic cortisol concentration.
Earwax samples were less likely to be influenced by confounding factors, such as short-term stressful events, and alcohol consumption, the researchers said.
"Cortisol sampling is notoriously difficult, as levels of the hormone can fluctuate, so a sample might not be an accurate reflection of a person's chronic cortisol levels," explained Dr Andres Herane-Vives from University College London's Institute of Cognitive Neuroscience, who led the study.
"Moreover, sampling methods themselves can induce stress and influence the results," he added.
Device Can Be Used at Home
Dr Herane-Vives has developed an earwax sampling device that can be used at home without clinical supervision.
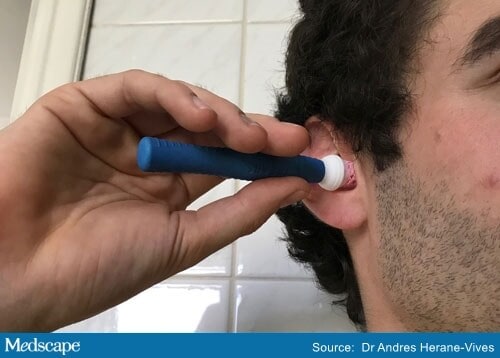
The researchers brought in 37 participants to test different cortisol sampling techniques.
Earwax from both ears was extracted using a standard syringe procedure that is commonly associated with local pain.
One month later, earwax from the left ear was obtained using the same procedure.
Meanwhile, samples of earwax from the right ear were obtained by the participants themselves using the novel device.
The researchers also analysed hair samples that represented the previous month's cortisol output, and serum samples that reflected the effect of systemic stressors on cortisol levels.
The study found that the self-sampling device did not increase cortisol locally. Also, it provided a cortisol level that was least likely to be affected by confounding factors over the previous month.
'Accurate and Affordable'
The authors said that self-obtained earwax samples might provide an alternative accurate but more affordable way of measuring chronic cortisol concentration.
Dr Herane-Vives said earwax had similar properties to another natural wax, honeycomb from bees, known for preservative properties and resistance to bacterial contamination. Those properties made it suitable for home sampling and the ability to be sent off for laboratory analysis with low risk of contamination.
Dr Herane-Vives said: "After this successful pilot study, if our device holds up to further scrutiny in larger trials, we hope to transform diagnostics and care for millions of people with depression or cortisol-related conditions such as Addison's disease and Cushing's syndrome, and potentially numerous other conditions."
The researchers said the test could be used to measure glucose levels, and potentially COVID-19 antibodies, accumulating in earwax.
Dr Herane-Vives has set up a company, Trears, to bring the device to the market with funding from the UCL Hatchery startup incubator.
Measuring Earwax Cortisol Concentration using a non-stressful sampling method. Heliyon. Published 4 November 2020.



