A breathing aid that could help treat people with COVID-19 and keep them out of intensive care units has been designed within a few days.
The Continuous Positive Airway Pressure (CPAP) unit was developed in response to a Government call for manufacturing firms to create ventilators to boost the NHS's ability to treat an expected surge in patients who develop breathing difficulties due to the virus.
The breathing aid would be built by Mercedes-AMG High Performance Powertrains, a British Formula 1 engine manufacturer based in Northamptonshire.
Reverse Engineering
Mechanical engineers at University College London (UCL) and clinicians at University College London Hospitals NHS Foundation Trust (UCLH) started work on the device on 18th March.
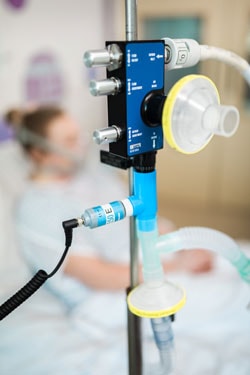
The team took 100 hours to 'reverse engineer' an existing device that could be produced rapidly and which would deliver oxygen to a patient's lungs without the need of a ventilator.
Prof Tim Baker from UCL Mechanical Engineering said: "Given the urgent need, we are thankful that we were able to reduce a process that could take years down to a matter of days.
"From being given the brief, we worked all hours of the day, disassembling and analysing an off-patent device. Using computer simulations, we improved the device further to create a state-of-the-art version suited to mass production."
CPAP [systems] were approved by the Medicines and Healthcare products Regulatory Agency for NHS use some years ago.
Similar devices have been used in China and Italy to help people with COVID-19 breathe more easily, when a passive oxygen supply was insufficient. Reports from Italy suggested that around half of patients given CPAP avoided the need for invasive mechanical ventilation. However, such devices are in short supply in UK hospitals, the UCL team said.
One hundred of the CPAP units were being delivered to UCLH for clinical trials.
Prof Mervyn Singer, a critical care consultant at UCL Medicine, said: "These devices will help to save lives by ensuring that ventilators, a limited resource, are used only for the most severely ill."
Andy Cowell, managing director of Mercedes-AMG High Performance Powertrains, said: “The Formula One community has shown an impressive response to the call for support, coming together in the 'Project Pitlane' collective to support the national need at this time across a number of different projects."
Project Pitlane comprises seven UK-based Formula 1 teams who have volunteered to divert their mechanical skills to helping to create medical devices for treating COVID-19 patients.
Prof Tim Cook, a specialist in anaesthesia and intensive care medicine at the Royal United Hospital Bath NHS Trust commented: "Anyone who can be kept out of ICU by using CPAP is a win-win-win for patient, healthcare system and staff."
Duncan Young, professor of intensive care medicine at the University of Oxford, added a note of caution: "The use of CPAP machines in patients with contagious respiratory infections is somewhat controversial as any small leaks round the mask could spray droplets of secretions on to attending clinical staff," he told the Science Media Centre.
Industry Co-operation to Find Solutions
In other initiatives, a consortium of technology and engineering firms in the UK have come together to produce ventilators that could be used in NHS hospitals.
The VentilatorChallengeUK consortium includes Airbus, BAE Systems, Ford, Rolls-Royce, and Siemens.
The consortium is led by Dick Elsy, CEO of High Value Manufacturing Catapult, a group of manufacturing research centres in the UK.
It said it was in a position to accelerate production of an agreed new design, based on existing technologies, which could be assembled from materials and parts in current production.
Meanwhile, vacuum cleaner manufacturer Dyson said it had received an initial order of 10,000 units of its CoVent ventilator
Work would start at its site at the former RAF base of Hullavington, the company said.
Entrepreneur Sir James Dyson told Forbes magazine: "Since I received a call from Boris Johnson 10 days ago, we have refocused resources at Dyson, and worked with TTP, The Technology Partnership, to design and build an entirely new ventilator."
Regulatory Exemptions
The UK is pursuing a three-pronged strategy for sourcing more ventilators.
Imports of machines from abroad would be combined with scaling up domestic production of existing designs and ordering new designs, The Guardian reported.
Firms were encouraged to submit proposals to build ventilators, or their components, by the Department for Business, Energy and Industrial Strategy.
Since industry was urged to bring its design and manufacturing prowess to bear, the MHRA issued guidance on exemption from the Medical Device Regulations. It involved fast-track approval of medical devices in which manufacturers would be required to seek approval of their devices before placing them on the market.
Among other companies increasing production of ventilators was Smiths Medical, whose CEO, Andrew Reynolds Smith, said: "We are doing everything possible to substantially increase production of our ventilators at our Luton site and worldwide."
Penlon, based in Abingdon, said it had "initiated plans and actions to ramp up our manufacturing capacity" of its Nuffield 200 Anaesthetic Ventilator.





